The Challenge of SDC Interoperability
The promise of SDC is compelling: devices from different vendors, working in harmony across clinical networks and ensuring a secure and reliable data flow to where it is needed.
Use-Cases Include:
• Seamless device-to-device communication in operating rooms and intensive care units
• Alert delegation between devices or to central systems (“Silent ICU”)
• Aggregation of data into unified clinical dashboards
• Remote control of devices in infectious or isolated clinical environments
• Integration with clinical decision support systems
By adopting SDC, medical systems become more modular, clinically safe, and future-ready for interoperability in connected healthcare. Yet achieving interoperability is not simple:
• Integration complexity: Testing SDC behavior across diverse devices requires significant setup and expertise.
• Hardware dependency: Many testing workflows depend on physical prototypes, slowing development cycles.
• Limited scalability: Validating performance across an entire network is often impractical until deployment.
For manufacturers and testing labs, these challenges translate into higher costs, slower time-to-market, and more risk when releasing new devices.
A Smarter Way to Test and Validate
The new SDC Integration for CANoe was built to address these barriers head-on. It is designed to make testing, validation, and simulation of SDC devices faster, scalable, and cost-efficient. It extends CANoe’s capabilities to the world of service-oriented medical device communication.
Whether you are simulating complex SDC networks, analyzing message flows, or validating device behavior under edge conditions - the solution gives you full control and visibility. Key capabilities include:
• Participant Simulation: Accurately simulate both providers and consumers, replicating how real devices interact in a clinical environment.
• Traffic Analysis: Monitor and analyze network traffic for deep insights into performance and compliance.
• Scripting Interface: Automate interactions with simulated participants, enabling reproducible and sophisticated test scenarios.
• Hybrid Testing: Connect seamlessly to actual devices and real SDC networks.
• Scalability: From a single device to entire clinical networks, the tool supports to every stage of development.
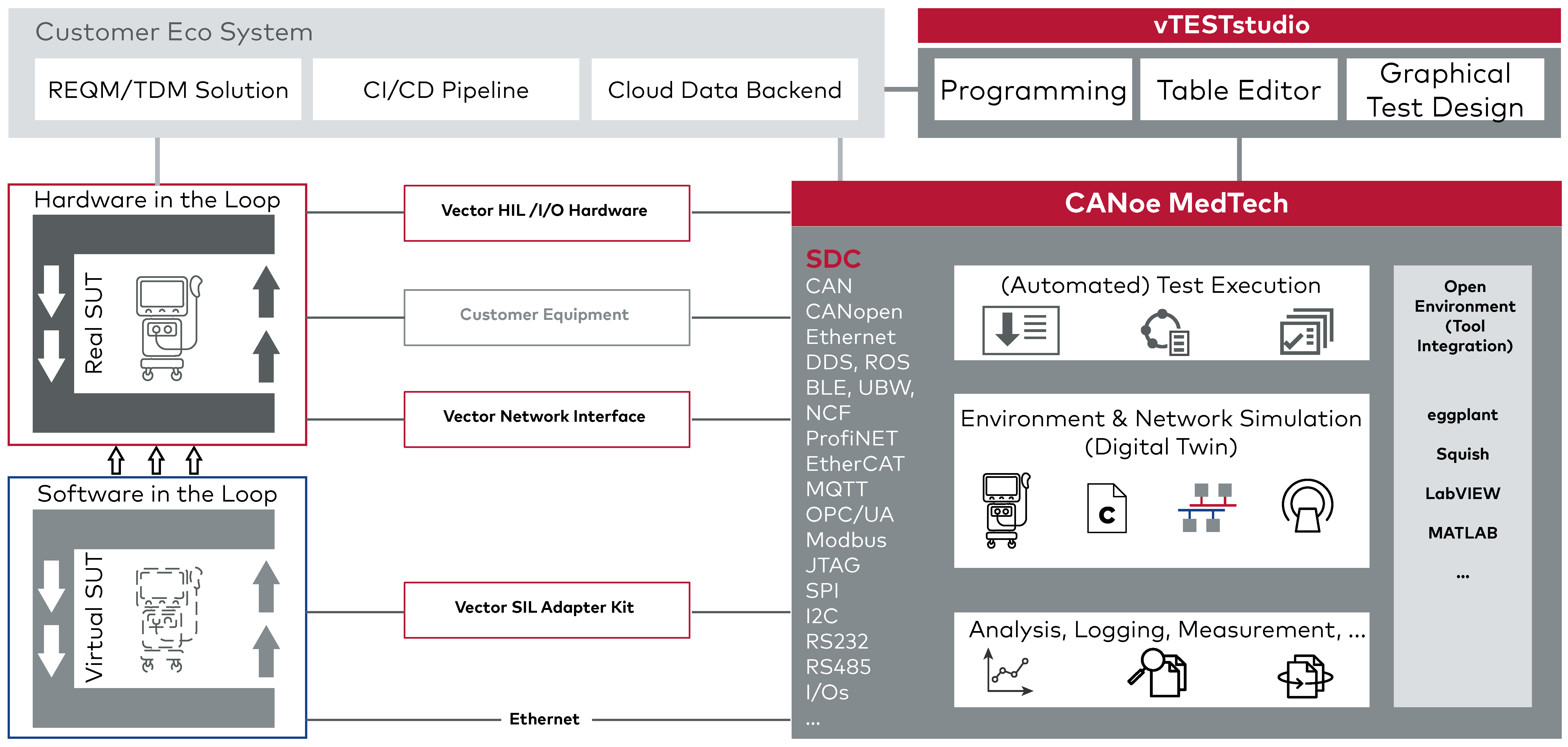
Part of a Proven, Versatile Testing Ecosystem
The SDC Integration is an integrated extension of CANoe, a powerful and established development and testing platform used across industries for validating communication networks and embedded systems.
It brings full support for service-oriented medical device communication, seamlessly integrated into a broader testing ecosystem that spans Ethernet-based protocols like EtherCAT, DDS, and OPC UA, serial communication interfaces such as UART, SPI, and I²C as well as digital and analog I/O.
The SDC Integration benefits from CANoe’s powerful features such as customizable test automation, graphical panels, detailed logging, and support for both software- and hardware-in-the-loop testing. With open interfaces and extensibility, CANoe allows integration into existing toolchains, CI setups, and laboratory environments.
For users in the medical technology field, this means you can build on a single, scalable platform - from early development through to integration and verification - with full SDC capability built right in.
From Simulation to Real-World Validation: Two Common Development Scenarios
But how does this look in practice? Let us explore two real-world development scenarios where CANoe proves especially valuable.
I. Integrating Legacy Medical Devices into an SDC Network
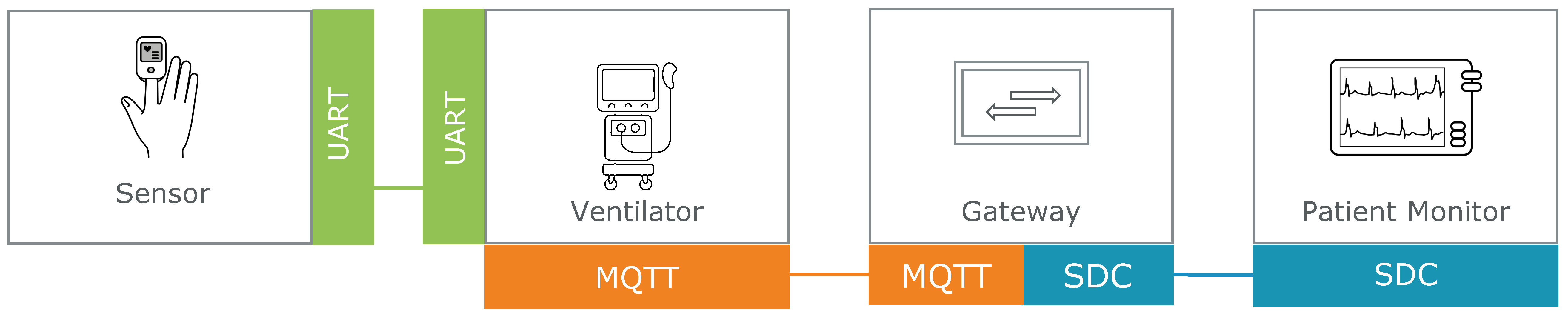
Hospitals often rely on established devices that were never designed for interoperability. To enable these devices to participate in networks alongside SDC-enabled equipment like patient monitors, a communication bridge is needed.
In this exemplary case, an SDC-MQTT gateway connects a legacy ventilator, which exposes data via MQTT, with an SDC network. This allows data exchange and coordinated behavior with other SDC-capable devices, without modifying the ventilator's original software or hardware.
1. Full Environment Simulation for Isolated Testing
Using CANoe with SDC, the complete system environment can be simulated - including both the legacy medical device and the network of surrounding SDC devices. This includes virtual models of the legacy ventilator (communicating via MQTT) and SDC-compatible equipment.
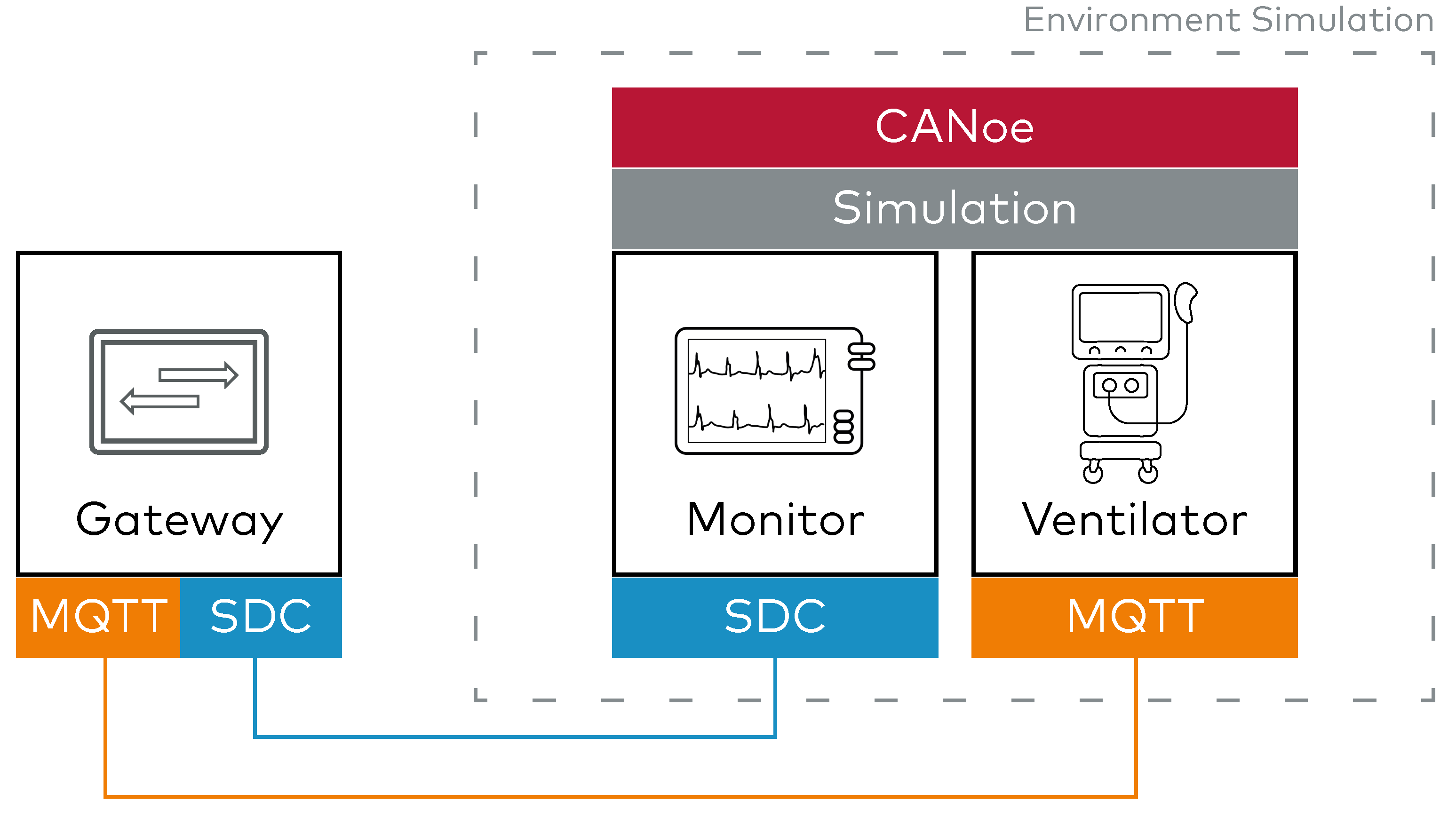
This simulation environment enables isolated development and testing of the gateway without the need for physical hardware. Gateway logic and data translation can be validated in a deterministic and reproducible setting, allowing for rapid development iterations, in-depth debugging, and flexible testing of complex scenarios.
Beginning with Software-in-the-Loop (SIL) development enables early detection of potential problems and can decrease the integration time required for the target gateway hardware.
2. Hardware-in-the-Loop (HIL): Real Device Stimulation and Feedback Analysis
After the gateway has passed isolated testing, the real ventilator can be integrated into the test setup. CANoe is configured to simulate sensor input to the ventilator (e.g., SpO₂ data via UART) and to monitor the resulting communication as it flows through the gateway into the SDC network.
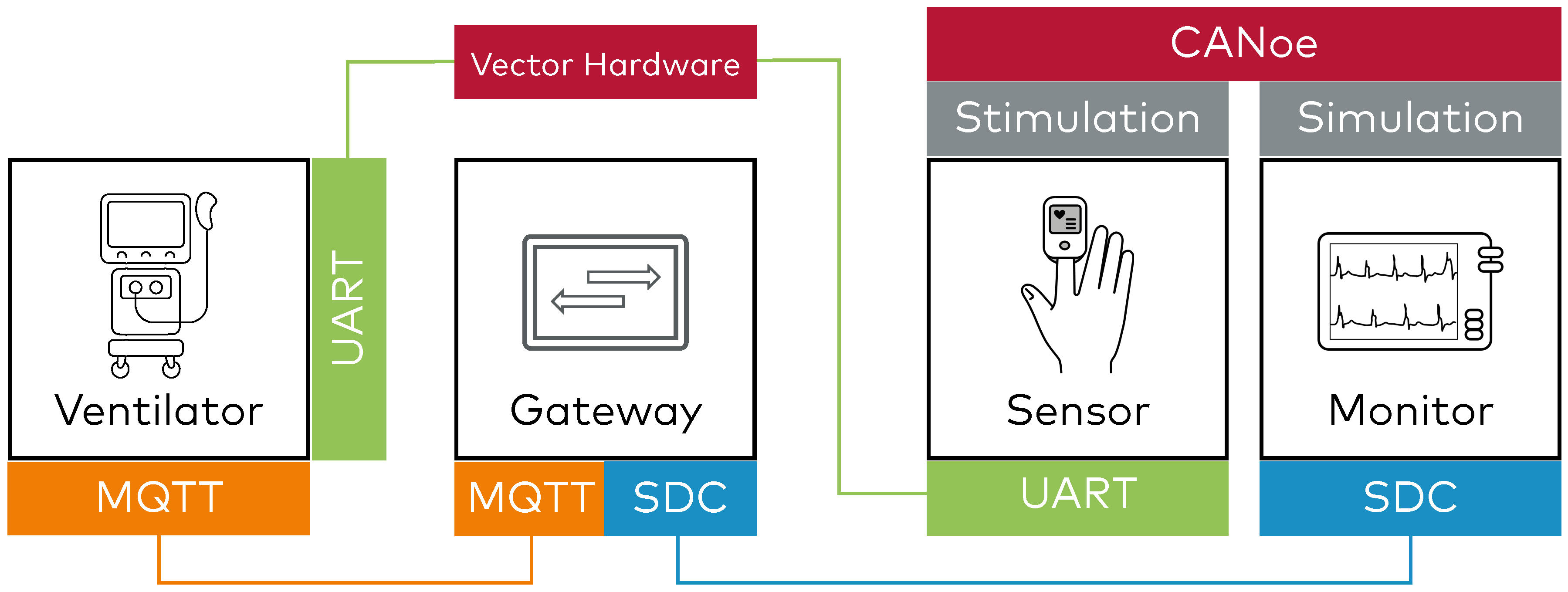
This setup enables full end-to-end validation of the integration, confirming accurate data translation and reliable transmission between the physical ventilator and SDC-compliant systems. It verifies bi-directional communication, ensuring messages are correctly mapped across protocols.
By simulating clinical scenarios under realistic conditions, CANoe helps developers identify timing issues, data mismatches, and edge-case behaviors early in the development cycle.
II. Developing Medical Devices with Native SDC integration
For innovators designing new medical devices with built-in SDC support, the challenge is ensuring seamless interoperability before the first prototype is even built.
Developing a networked medical device such as an SDC-enabled infusion pump, requires rigorous validation, from functional behavior to real-world interoperability. Without the right tools, developers often wait until late in the project to test device behavior against real networks.

Using CANoe, developers can move from full simulation to hardware integration in a structured workflow that accelerates development, streamlines integration, and reduces risk throughout the validation process. This example outlines a three-step approach to develop and test the infusion pump.
1. Full Simulation: Early Use-Case Evaluation
During the initial phase, the infusion pump is implemented as a fully virtual SDC device within CANoe. Simulated models implement functional behavior, the SDC service interface, and communication patterns. The surrounding SDC network environment, including other peer devices, is also replicated in CANoe to facilitate comprehensive evaluation of interaction flows and interoperability.

This setup allows for early and fast prototyping of distributed SDC use-cases and validation of the device interaction in potential clinical networks.
2. Software-in-the-Loop (SIL): Evaluate Real SDC Software within a Simulated System
Following successful validation of the initial design in simulation, the actual software stack for the infusion pump is deployed, comprising the SDC stack, device application logic, and service handlers. This software operates on a PC or development platform, with CANoe continuing to simulate the SDC network and interactions previously established during full simulation.
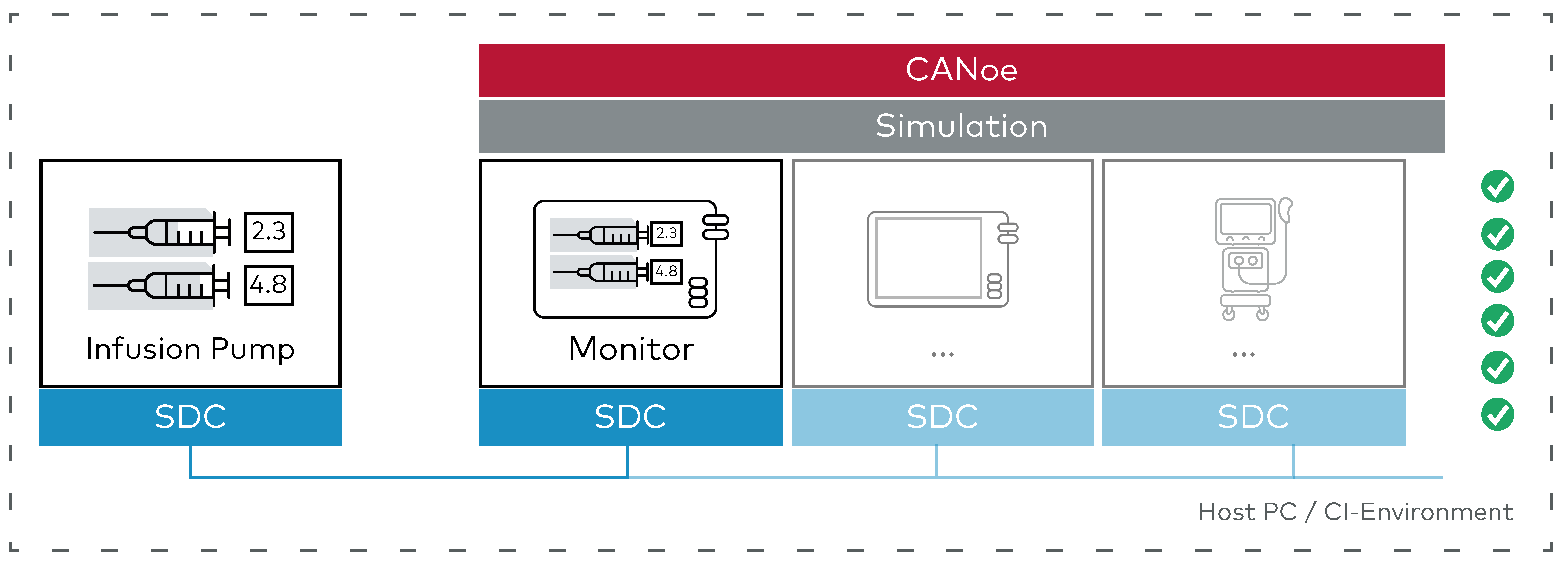
Previously established workflows and test scenarios can be repeated with the actual device software connected. This setup allows for detailed observation of device interactions and communication and supports rapid iterations on the System under Test software.
At this stage, CANoe can also be seamlessly integrated into Continuous Integration and Continuous Testing (CI/CT) pipelines, enabling automated regression testing and continuous verification of SDC communication behavior as software evolves. This ensures consistent quality and faster feedback loops throughout thedevelopment lifecycle.
3. Hardware-in-the-Loop (HIL): Comprehensive Device Validation
In the final phase, the physical infusion pump hardware is connected. CANoe maintains the simulation of the SDC network and peer devices, enabling direct interaction with the real device via its native communication interfaces.

All simulation and test procedures developed in the early simulation and SIL stages remain applicable when integrating the actual device. This continuity ensures consistency throughout all testing phases and streamlines the overall integration process.
Benefits that Drive Value
CANoe with SDC is not just a testing framework, it is an accelerator for the adoption of SDC across healthcare.
• Faster development cycles: Validate designs early, whether building gateways for legacy systems or designing new devices.
• Lower costs: Replace extensive hardware setups with high-fidelity simulations.
• Smooth transition from SIL to HIL: Start in virtual environments, then validate seamlessly with real hardware.
• Scalable validation: Move from single device to full clinical network testing with ease.
• Confidence in compliance: Ensure devices meet interoperability requirements early in development.
• Streamlined certification: Ensure regulatory readiness with traceable test evidence.
Building the Future of Interoperable Healthcare – with CANoe.SDC
As clinical environments grow more complex, the demand for safe, reliable, and interoperable device communication only increases. The IEEE 11073 SDC standard lays the foundation, but adoption depends on practical, effective tools.
CANoe with its new SDC integration fills that gap — giving manufacturers, integrators, and testing labs the ability to test smarter, scale faster, and bring truly interoperable devices to market with confidence.
Ready to take the next step in SDC interoperability testing?
Click here to learn more about CANoe MedTech or get in contact to discuss your challenges.




