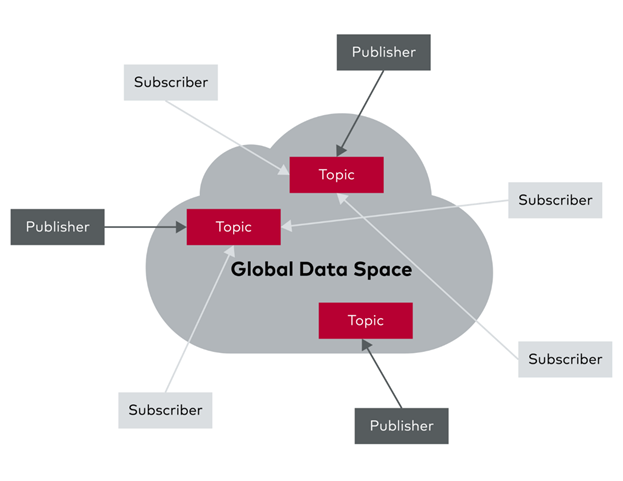
The Core Concept: A Global Data Space for Medical Devices
At its heart, DDS is a middleware standard for data-centric, publish-subscribe communication. Instead of devices directly addressing each other, they publish data to a shared "global data space" on specific topics.Other devices can then subscribe to these topics to receive the data they need. This decoupled approach virtually eliminates complex network programming for distributed applications. Imagine a patient monitoring system where a bedside monitor publishes vital signs. Multiple other systems, like a central nursing station, an electronic health record (EHR) system, and even a remote specialist's tablet, can all subscribe to and receive this data in real-time without the monitor needing any knowledge of their existence or location.
SDC and DDS: Competing or Complementary?
When discussing interoperability in medtech, the Service-oriented Device Connectivity (SDC) standard, part of the IEEE 11073 family, often enters the conversation. SDC is specifically designed to enable manufacturer-independent, point-of-care medical device-to-device communication and integration with clinical information systems. It's built on a service-oriented architecture using web services, enabling bidirectional data exchange and even remote control of devices.
While both DDS and SDC aim to solve the interoperability challenge, they approach it from different architectural philosophies. DDS utilizes a data-centric publish-subscribe model, ideal for scalable, real-time data sharing in highly dynamic and large-scale systems. In contrast, SDC is more service-oriented, focusing on discoverable services and interactions between specific point-of-care devices. Some view DDS as potentially too complex for simpler medical device operations where the extensive Quality of Service (QoS) management might be overkill. However, the two are not necessarily mutually exclusive. A healthcare system could potentially leverage both: SDC for interoperability in the highly regulated point-of-care environment, and DDS for high-throughput, real-time data distribution for applications like medical imaging or large-scale physiological data logging across the hospital network. The choice between them, or a decision to use them in concert, depends on the specific requirements of the medical application, from the need for real-time performance and scalability to the specific device types and regulatory constraints.
The Transformative Benefits of DDS in Medtech
The adoption of DDS in medical technology brings a wealth of advantages for developers and, ultimately, for patient care.
- Real-Time Performance and Scalability: DDS is designed for high-performance, low-latency data exchange, which is critical in life-or-death medical scenarios. Whether it's streaming high-resolution medical imaging data or coordinating the precise movements of a surgical robot, DDS can handle massive data flows efficiently. Its architecture is inherently scalable, allowing for the addition of new devices and applications to the network without disrupting existing operations.
- Enhanced Interoperability: A major challenge in healthcare is the integration of heterogeneous devices from various manufacturers, which often use proprietary communication protocols. DDS addresses this by providing a standardized, platform-independent communication layer. This "plug-and-play" capability simplifies the integration of new devices, reducing development costs and enabling more cohesive and comprehensive patient monitoring and treatment systems.
- Improved Reliability and Robustness: Medical devices must be exceptionally reliable. DDS offers extensive Quality of Service (QoS) policies that allow developers to fine-tune data delivery to meet specific requirements for reliability, durability, and latency. This ensures that critical data is delivered when and where it's needed, even in the face of network fluctuations.
- Inherent Security: The confidentiality and integrity of patient data are non-negotiable. The DDS standard includes a security specification that provides for authentication, access control, and encryption, helping to safeguard sensitive medical information.
Navigating the Challenges of DDS Implementation
Despite its numerous benefits, integrating DDS into complex medical devices is not without its challenges.
- Complexity of the Standard: DDS is a powerful and flexible standard, but this also means it can be complex to master. Configuring the numerous QoS parameters to achieve the desired behavior requires a deep understanding of the protocol. Incorrect configuration can lead to suboptimal performance or unexpected behavior.
- Interoperability Nuances: While DDS promotes interoperability, issues can still arise between different vendor implementations or even different versions of the standard. These incompatibilities can manifest in subtle ways, making them difficult to diagnose without the right tools.
- Security Implementation: Implementing the security features of DDS requires careful planning and execution. Ensuring that all devices on the network are properly authenticated and that data is encrypted correctly is a significant undertaking.
- The Testing Conundrum: Thoroughly testing a distributed DDS-based system can be incredibly challenging. Simulating a large-scale network with numerous publishers and subscribers, each with its own unique QoS requirements, is often impractical with physical hardware alone. Identifying and isolating issues within this complex web of interactions can be a time-consuming and frustrating process for development teams.
The Solution: Robust Testing and Simulation
This is where our expertise as a provider of test and simulation tools comes into play. We understand that to harness the full power of DDS, developers need to be able to rigorously test and validate their implementations. Vector has therefore put high effort in adding the new Option CANoe.DDS to the CANoe product family. (Simulation, Development and Test of DDS Systems | Vector) Our tools empower you to:
- Simulate Realistic Scenarios: Create virtual environments that mimic the complexity of a real-world clinical network. This allows you to test your device's behavior under a wide range of conditions, with hundreds or even thousands of simulated DDS participants, without the need for extensive hardware setups.
- Automate Testing: Automate repetitive and complex test cases to ensure consistent and thorough validation of your DDS implementation. This frees up your development team to focus on innovation while ensuring that any changes to the system don't introduce new bugs.
- Analyze and Debug with Precision: Gain deep insights into the DDS communication on your network. Our tools provide detailed analysis of DDS traffic, allowing you to quickly identify and resolve issues related to QoS settings, interoperability, and security.
- Validate Security: Test the robustness of your security implementation by simulating various attack vectors and ensuring that your data remains confidential and secure.
By embracing a comprehensive testing and simulation strategy, developers can overcome the challenges of DDS implementation and unlock its full potential. The result is more reliable, interoperable, and secure medical devices that can truly transform patient care. As the medtech landscape continues to evolve, a robust and well-tested DDS implementation will be a key differentiator for any device manufacturer.